Crop diseases cause about loss of ~ $ 220 billion annually. Controlling of crop diseases without compromising agricultural traits has been a long-sought dream of crop breeders. A key to this end is to identify conserved and natural immune signaling molecules with a broad spectrum of pathogen resistance activity. Pathogens exude pathogenic factors known as pathogen effectors into plant cells during infection. Detection of the secreted pathogen effectors in plants is mainly through a large family of proteins or immune receptors termed NLRs and leads to triggering of plant immunity. TIR-NLRs (TNLs) constitute one subfamily of NLRs and detection of pathogen effectors activates the enzyme activity of TNLs encoded within their shared TIR moiety. The TIR-encoded enzyme activity is evolutionarily conserved and important for plant immunity. It was assumed that enzyme products are responsible for triggering of TNL immunity. However, their identifies remained elusive.
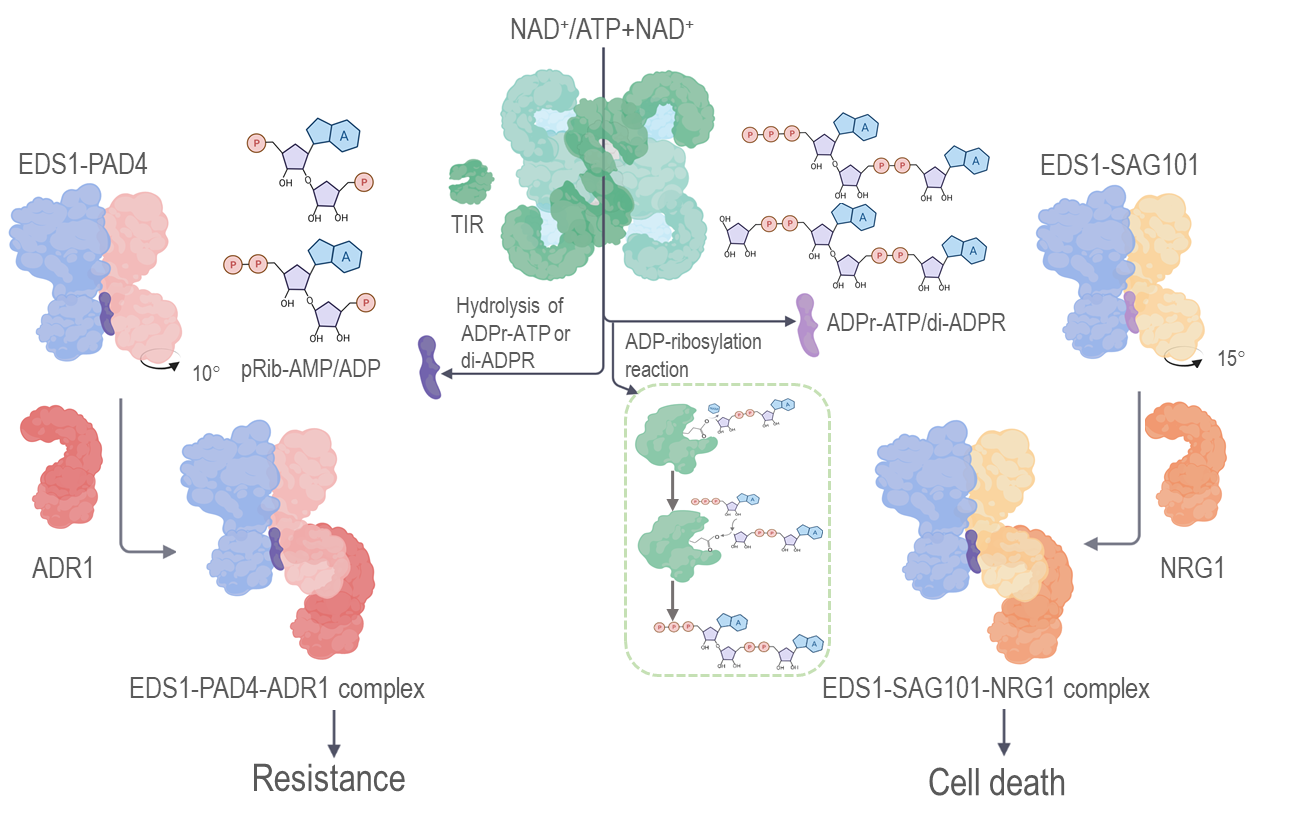
Figure 1. TIR-catalyzed small molecules controlling two immunity branches
Previous researches from Prof. Jane Parker and other laboratories has established that TNL-mediated immune response depends on plant-specific lipase-like EDS1 family members. In Arabidopsis, EDS1 forms constitutive heterodimers with its paragloues PAD4 or SAG101 to mediate immune signaling. The EDS1-PAD4 and EDS1-SAG101 dimers, presumably through recognition of TIR-encoded enzyme products, interact with and activate downstream components ADR1s and NRG1s, respectively. In these two papers, the Chai group successfully reconstituted activation of ADR1 and NRG1 by a TNL resistosome in insect cells. The authors showed that TIR proteins from different plant species catalyze production of small molecule, which bind to EDS1-PAD4 and EDS1-SAG101 for activation of ADR1 and NRG1, respectively. The identities of the EDS1-PAD4- and EDS1-SAG101-bound small molecules were determined to be pRib-ADP/AMP and ADPr-ATP/ADPR by structural biology and mass spectrometry. The identities of the small molecules were confirmed by chemically synthesized compounds which have similar activity in activating EDS1 dimer interactions with ADR1 and NRG1. Structural data revealed that the TIR-catalyzed small molecules activate EDS1 dimers through a conserved allosteric mechanism. In addition to the known NADase activity, the authors found that a novel enzyme activity of TIRs called ADP-ribosylation catalyzes production of ADPr-ATP/ADPR and pRib-ADP/AMP. Identification of TIR-catalyzed small molecules fills a missing link in EDS1 signaling pathway. The data from the two studies support the TIR-catalyzed products as novel second messengers of plant immunity.
Prof. Jijie Chai from School of Life Sciences, Tsinghua University and University of Cologne, and Prof. Jane E. Parker from the Max Planck Institute for Plant Breeding Research, Prof. Junbiao Chang from Zhengzhou University and Henan Normal University, and associate professor Zhifu Han from Tsinghua University are the co-corresponding authors of the two papers. Shijia Huang and Aolin Jia, graduate students from the School of Life Sciences of Tsinghua University, Wen Song and Giuliana Hessler, postdoctoral fellows at Max Planck Institute for Plant Breeding Research, and Dr. Yonggang Meng, fellow in Zhengzhou University are the co-first authors of the first article. Aolin Jia and Shijia Huang, Dr. Wen Song and Junli Wang, postdoctoral fellow at Max Planck Institute for Plant Breeding Research, Dr. Yonggang Meng, and Yue Sun, graduate students at the School of Life Sciences of Tsinghua University, are the co-first authors of the second article. Dr. Yu Xiao, Dr. Shoucai Ma from the School of Life Sciences of Tsinghua University, Dr. Dongli Yu, Dr. Ertong Li, Dr. Huanhuan Sun, Dr. Henriette Laessle, Dr. Jan Jirschitzka from the Max Planck Institute for Plant Breeding Research, Jan Gebauer, Ulrich Baumann from University of Cologne, Dr. Jiao Hou, Dr. Ruiqi Liu, Dr. Tiantian Zhang, professor Wenquan Yu from Zhengzhou University all contributed to this research. Lina Xu and associate Researcher Xiaohui Liu from National Protein Science Facility, Tsinghua university also contributed to this research especially on identifying small molecules. The National Center for Protein Science (Beijing), the cryo-electron microscope platform of Tsinghua University, the high-performance computing platform of Tsinghua University and the Shanghai Synchrotron Radiation Light Source provided equipment and technical support for this research. These researches were supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, Beijing Advanced Innovation Center for Structural Biology, Tsinghua University-Peking University Joint Center for Life Sciences, the Alexander von Humboldt Foundation, the Max-Planck-Gesellschaft, Deutsche Forschungsgemeinschaft.
http://doi.org/10.1126/science.abq3297
http://doi.org/10.1126/science.abq8180








